To complement the private group that we have had running on Linkedin for some time, we have now opened up a public ‘company page’.
You may follow us there!

 Dr Richard FitzGerald, Royal Liverpool University Hospital.
Dr Richard FitzGerald, Royal Liverpool University Hospital.Proceedings from AHPPI’s conference on Risk Management in Early Phase Clinical Trials, 29 Oct 2015 (link).
Starting with TeGenero and referring to subsequent guidelines for Phase I trials from such bodies as MRC, ABPI, expert scientific groups etc., Richard went on to describe the risks of clinical trials of various types of antibody, not all of which are monoclonal. He did, however refer specifically to mAbs, their therapeutic potential and the fact that several are currently marketed. With a comparison of small molecules and mAbs he argued that the latter may be safer due to being VERY target specific with NO off target effects and a simpler and predictable metabolism. However, by comparison, size and molecular complexity may lead to risk as there may be many target characteristics and expressions as well as targets which are cell based.
Potential immunogenicity has always been a risk and raises certain questions whereby preclinical programmes need to be optimized in order to support relevant clinical development especially with respect to integrated safety pharmacology: in terms of toxicology Richard showed the vast range of studies that have already been performed on marketed compounds, ranging from two weeks in mouse to 9 months in monkeys!
The risks of cytokine release syndrome were also stressed and this led on to consideration of the first dose to be given. The use of MABEL was stressed and it was pointed out that TGN 1412 has now been given safely to man in doses considerably lower than originally. Immunogenicity, cross reactivity and pre-existing antibodies were all discussed and the risks and benefits of healthy volunteers and patient groups explored with evidence from a meta analysis of first in man studies in an attempt to quantify risk. This was a wide-ranging and fascinating summary of the state of current knowledge.
Written by Dr Peter Dewland, Peter Dewland Consulting. Peter is an independent  consultant in pharmaceutical medicine and has a Master’s degree in Medical Ethics together with degrees in Biochemistry and Medicine along with 30 years experience of developing new drugs. He is a founder member of the Faculty of Pharmaceutical Medicine and now both teaches and examines students of this specialty. He is a past Chairman and current Treasurer of the AHPPI.
consultant in pharmaceutical medicine and has a Master’s degree in Medical Ethics together with degrees in Biochemistry and Medicine along with 30 years experience of developing new drugs. He is a founder member of the Faculty of Pharmaceutical Medicine and now both teaches and examines students of this specialty. He is a past Chairman and current Treasurer of the AHPPI.
 Dr John Lambert, PAREXEL.
Dr John Lambert, PAREXEL.Proceedings from AHPPI’s conference on Risk Management in Early Phase Clinical Trials, 29 Oct 2015 (link).
John introduced his talk by describing the changing face of early phase clinical trials and the potential for ‘risk’ in studies that demonstrate an ever-increasing complexity in their design. The regulatory environment and requirements for risk evaluation and mitigation strategies were discussed.
John gave a fascinating insight into how PAREXEL, as a global CRO, addresses the various challenges. An insight was also given into the best way to navigate the regulatory and safety hurdles through a formal process of risk assessment for each and every study, with emphasis on being placed on first in human (FIH) studies.
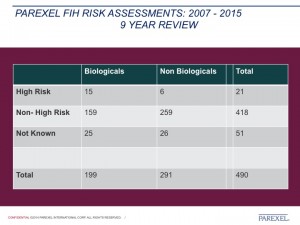
The second half of the presentation focused on risk mitigation strategies and how PAREXEL address the practical aspects of the design and staffing of FIH studies. John closed his presentation by sharing the risk assessment statistics on the number of FIH studies conducted by PAREXEL between 2007 and 2015. The talk stimulated a lively discussion between the speaker and the audience.
Written by Dr Tim Hardman. Tim is the Managing Director of Niche Science and
Technology Ltd., a CRO that has been working with industry and academia since 1998. Tim has a PhD from Imperial College, London, and degrees in Physiology and Pharmacology, along with over 20 years experience of developing new drugs. He is also a member of the AHPPI Secretariat.
The presentations from this year’s AHPPI Annual Meeting have been uploaded to the conference page (link).
We are pleased to announce the programme for this year’s AHPPI Annual Meeting (link).
There will be a range of speakers from commercial and academic backgrounds delivering presentations on a variety of issues within this important theme.
AHPPI members and non-members are welcome.
Delegates can expect opportunities to network with early stage clinical research colleagues and hear about the new developments and plans for AHPPI.
We are pleased to announce this year’s AHPPI Annual Meeting.
There will be a range of speakers from commercial and academic backgrounds delivering presentations on a variety of issues within this important theme.
AHPPI members and non-members are welcome.
Delegates can expect opportunities to network with early stage clinical research colleagues and hear about the new developments and plans for AHPPI. More details will be shared soon.
Save the date now!
Recently, the FDA, other regulatory bodies, and pharmaceutical companies have been exploring options to replace TQTs with preclinical testing or with data collected during standard Phase I trials.
Join colleagues from the FPM and also the British Pharmacological Society and Association for Human Pharmacology in the Pharmaceutical Industry to debate the current scientific, regulatory and safety issues around TQT studies and potential alternatives.
Click here for further information or visit the Faculty of Pharmaceutical Medicine website to register.
The European Federation for Exploratory Medicines Development (EUFEMED), a newly formed Federation of Early Phase associations; Club Phase 1 (France), BAPU (Belgium), AGAH (Germany) and AHPPI (UK), proudly hosted the 3rd Joint Conference of European Human Pharmacological Societies, which took place 21st and 22nd May 2015 in Brussels.
Delivered by an excellent panel of key industry representatives, the meeting provided an overview on Europe’s current global market position, advances in European clinical trial regulation and current issues in exploratory clinical drug development, attracting a wide audience of scientific, regulatory and academic professionals across Europe.
Following the recent stakeholder meeting on the transparency addendum for the EU portal and EU database, which took place at the EMA on 01 June 2015, the Exploratory Medicines Development (EUFEMED) and the European CRO Federation (EUCROF) have made a joint submission to the European Medicines Agency (EMA) on the timelines for publication of summary results for trials without therapeutic or prophylactic intent (“Category 1 trials”).
Please find below submission as sent to the EMA earlier today;
EUFEMED EUCROF letter to EMA 15 Jun 2015
EUFEMED survey on publication of clinical trial results final report 10-06-2015
EUCROF Position Paper Public Access to Early Phase EU database information 31 OCT 2014
The EMA recently launched a public consultation on their “Draft proposal for an addendum, on transparency, to the “Functional specifications for the EU portal and EU database to be audited – EMA/42176/2014”, for proposals and options on the application of exceptions in relation to the transparency provisions of the European Clinical Trial Regulation.
Following the review of the EMA’s proposals, the AHPPI have submitted a joint response to the consultation together with all associations incorporated into the European Federation for Exploratory Medicines Development (EUFEMED).
Click here to download the response
AHPPI committee members actively participate in the ongoing discussions and stakeholder meetings on a European and UK level. Any additional feedback and support from our members is most welcome.