Steffan Stringer, published 23-Dec-2015. Correspondence steffan.stringer@alwynconsulting.co.uk.
Steffan Stringer, published 23-Dec-2015. Correspondence steffan.stringer@alwynconsulting.co.uk.
©2015 Steffan Stringer, Alwyn Consulting, www.alwynconsulting.co.uk
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
A survey methodology showed the meeting to be well organised, and good value for money. The venue was good but the facilities were not sufficient for the breaks. A longer meeting would improve networking opportunities and allow more time per speaker. Most attendees (63.64%) supported the AHPPI (Net Promoter Score loyalty metric).
Background
The Association for Human Pharmacology in the Pharmaceutical Industry (AHPPI) held its annual meeting on 29-Oct-2015. The meeting was titled Risk Management in Early Phase Clinical Trials and took place at the Academy of Medical Sciences in London. The meeting cost £35 per person, started at 12:30 and finished at 17:00 and was followed by a drinks reception. A survey was sent to the attendees the day after the meeting. Links to the presentations and an attendance certificate were provided to the attendees by follow-up emails.
Methods
A 10 question survey was constructed prior to the meeting using SurveyMonkey. The survey was intended to determine the level of satisfaction the attendees experienced and to solicit feedback for the planning and development of future meetings. The questions were all pulled from an existing library of questions and were either Eventbrite or SurveyMonkey certified.
The questions were as follows:
- Overall, how would you rate the event?
- What did you like about the event?
- What did you dislike about the event?
- How engaging were the speakers at the event?
- How organized was the event?
- Was the event length too long, too short, or about right?
- How would you rate the value for the money of the event?
- How could future events be improved? Select all that apply.
- Is there anything else you’d like to share about the event?
- How likely is it that you would recommend the AHPPI to a friend or colleague?
Questions 2, 3 and 9 collected free text responses. All other questions required the selection of a single or multiple predefined responses or a rating upon a 10-point scale. Questions and answers were not edited and were presented verbatim from the library of question/answer combinations. The survey did not collect any identifying data from the respondents. The survey remained editable until it was submitted. Upon completion of the survey, a respondent was able to see the aggregated and summarised data of previous respondents. The survey was issued to the 57 attendees at approximately mid-day on 30-Oct-2015 and closed on 08-Nov-2015. The recipients included the speakers, AHPPI committee members and the organising committee for the meeting.
The full survey page can be seen here (link to SurveyMonkey Web site).
Results
Twenty responses were received on the first day the survey was opened and the last response was collected 04-Nov-2015, approximately four business days after it was opened. A total of 33 responses were collected from the 57 invitations to participate (57.9%), 32 in response to an email invitation and one in response to a link to the survey. Questions 1, 5, 6 and 10 show results compared to SurveyMonkey global benchmarks. Where available, box and whisker plots have been chosen to display the numerical data rather than frequency histograms.
For free text responses, the verbatim responses are presented below. Each bullet point represents the complete response of one individual. Some typographical errors have been corrected and minor formatting changes made.
The full results dataset can be found here (link to SurveyMonkey Web site).
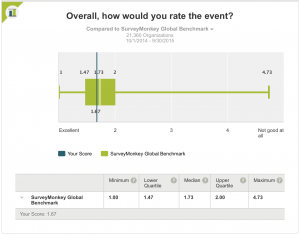
1. Overall, how would you rate the event?
The score was 1.67, better than the Global Benchmark median (1.73).
- What did you like about the event?
Twenty-eight respondents answered this question. Five skipped the question.
- Variety of content
- All speakers provided clear, concise and relevant information. Meeting was also well organised with good professional and time for social interactions
- Risk Management for Early Trials in biological -the speaker was very good balancing science and information
- High quality presentations. Interesting mix. Nice venue, it had a professional feel to it
- High standard of presentations. Wine & snacks afterwards!
- Topical theme. Speakers from differing backgrounds
Length of time for talks. Good opportunity for networking. Good quality of food and drinks - Topics, speakers, organisation
- Engaging, knowledgeable speakers. Good venue as ever. Kept to time
- The members- very friendly and knowledgeable. The presentations – very interesting and informative. The venue- small but cosy. The drinks reception – nice touch!
- Talks were interesting and good networking opportunities
- There was a variety of talks; it was relatively small which made networking easier; well organised and kept to time.
- Nice mix of people, good presentations, people open to networking
- Updates related to regulatory environment and new trends or changes in standard of practice (e.g. TQT). Meeting point
- Good attendance, agenda shared ahead of the meeting, most presentations were well thought out and informative
- It was good to see that the organization has positive plans for the future.
- In a half-day, some of very important topics in clinical trials are discussed.
- That there was a range of topical items discussed
- Opportunity to network – great topics discussed – brilliant organisation
- Good talks, nice to meet some old friends
- Topic content of risk management.
- Very relevant to my current job
- Chance to network. Interesting presentations
- Very well organized, good location and great facilities
- The agenda – the theme was very relevant and the talks all very informative and excellent. The name badges were good and big so they were easy to read!
- Talks were interesting the presenters were good (especially the second talk)
- The focus on updates on a number of essential topics relevant to drug development.
- The talks were well prepared, relevant and very engaging.
- The breadth of topics discussed and the active participation. The speakers were excellent, I thought in detailing the important issues.
3. What did you dislike about the event?
Twenty-five respondents answered this question. Eight skipped the question.
- Over run of some talks
- Meeting room limited in space although adequate
- The room was too dark during presentation – difficult to write notes. General concern with proposal of using other social media for sharing information and especially accessing the presentations
- A little crammed. The meeting format (n=50) and the relatively small room did not go well with the high level talks. Also some presentations would have warranted more time – for discussion
- Use a more comfortable space to host the event
- Room too hot
- Nothing, except perhaps biscuits at the start!
- Slides not given as notes to annotate
- Nothing!
- Nothing
- When the focus of a presentation is more business related rather than knowledge sharing
- Very much a lecture style, not the environment to network (it appeared most ppl were speaking with prior connections), very phase 1 unit focused (not a bad thing but perhaps more input from the Sponsor side would’ve been good)
- I thought it a pity that the speakers had too little time to present their slides, or too many slides to present in the short time, i.e. didn’t prepare too conscientiously. The result was that the pace was pretty hurried.
- Nil
- Too short
- Nothing
- Timing of presentations the speakers could keep to time better so that all slides can be seen. Suggestion – one speaker per presentation 15 mins.
- None
- Finishing when the queue to get into Oxford Circus tube station tailed back onto the street, and for Piccadilly tube station filled the station. I guess walking to Waterloo was good for me but I missed the train I was after!
- The coffee room got a bit hot before the presentations started. Lecture theatre was fine.
- Nothing. Central London location was good for me, half day was perfect as I got 4 hours work done first, drinks afterwards were very pleasant.
- The room with coffee, etc was a bit small for the number of attendees
- The narrow lounge where colleagues had a tea/coffee. It felt too cramped in there.
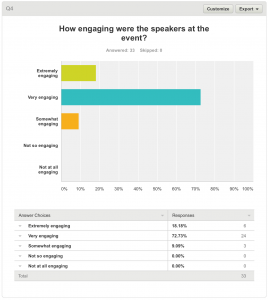
4. How engaging were the speakers at the event?
All respondents answered this question. Thirty respondents (90.91%) felt that the speakers were extremely or very engaging. Three (9.09%) felt the speakers were somewhat engaging.
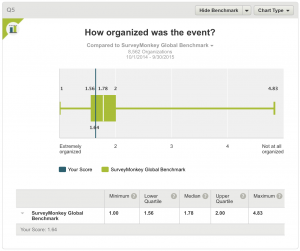
5. How organized was the event?
All respondents answered this question. The result, a score of 1.64 compares with a median of 1.78 in the Global Benchmark indicating that the meeting was well organised.
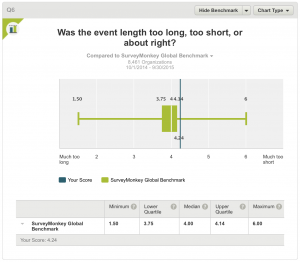
6. Was the event length too long, too short, or about right?
All respondents answered this question. The result, a score of 4.24 compares with a median of 4.00 in the Global Benchmark indicating that the meeting was shorter than the attendees would have liked.
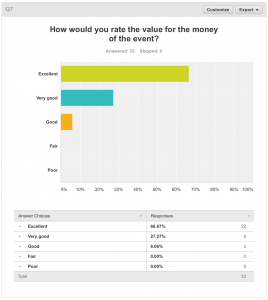
7. How would you rate the value for the money of the event?
All respondents answered this question. The event cost £35. 31 (93.94%) felt that the event was either Excellent or Very Good value for money. One respondent (6.06%) felt the event was Good value for money.
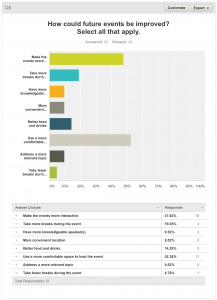
8. How could future events be improved? Select all that apply.
Twenty-one respondents answered this question. Twelve respondents skipped it.
9. Is there anything else you’d like to share about the event?
Seventeen respondents answered this question. Sixteen respondents skipped it.
- None
- I had hoped to get some more update on the regulatory view on the update of E14 or S7B
- If the event expands in size the venue would not suffice
- Appreciate the committee members hard work in organising a good event.
- Good to have representatives from Regulatory and ethic bodies
- It was really great. Above comments are no criticism just suggestions.
- The topics covered may have warranted a 1-day event. Some of the speakers where a little rushed. On the other hand, I think all the key messages came across very clearly.
- Very difficult to improve on this level of quality
- Uniformly excellent. Several of our staff will be joining the AHPPI.
- Have a slightly bigger room for food and drinks if possible. Got quite claustrophobic
- Excellent
- Would it be possible to have access to the presentations? Since some had reference to very interesting publications
- The event was good but i think there needs to be a focus on where the industry is going i.e. future innovation in clinical trials. Also more transparency with list of attendees and opportunity to interact/make new connections
- Giving a certificate to attendees are not a bad idea.
- None
- Longer notice of the date/programme would help, I think this was just 7 weeks.
- First one I have been to in a few years, very good and well organized, would be nice to have access to a list of attendees at the event on the day but don’t know if this is for confidentiality reasons this is not shared.
- Get it more widely advertised & make it inclusive to all colleagues working in different areas of the drug development process in Academia and Pharmaceutical Industry.
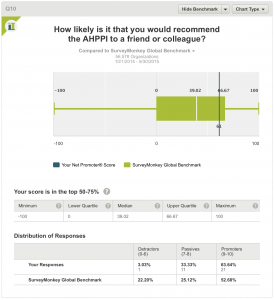
10. How likely is it that you would recommend the AHPPI to a friend or colleague?
Twenty-one (63.64%) respondents are promoters of the AHPPI, 11 (33.33%) are passives and one (3.03% )is a detractor. This compares favourably with Global Benchmarks – 52.68, 25.12 and 22.20% respectively.
Conclusion
The survey respondents reported that the meeting was well organised, but could have been longer in order to do justice to the content. They provided feedback that the venue was good but the facilities the committee had selected were not sufficient for the breaks. There is a sentiment that a longer meeting with larger facilities would enhance networking opportunities. The meeting was regarded by the majority as either excellent or very good value for money. Most attendees (63.64%) asserted they were net promoters of the AHPPI.
Conflicts of Interest
The author was on the organising committee for the meeting described in this article.
Acknowledgements
The author would like to thank Kerry Broomfield, Tim Hardman and Nathan McCavery for their support in organising and conducting the meeting. The AHPPI Committee would like to thank Niche Science & Technology and BioKinetic Europe for sponsoring the meeting.