 Dr Jörg Täubel, EUFEMED
Dr Jörg Täubel, EUFEMED
Proceedings from AHPPI’s conference on Risk Management in Early Phase Clinical Trials, 29 Oct 2015 (link).
Jörg explained that the AHPPI is a founder member of EUFEMED, which was born after a long gestation on 20 May 2015 in Brussels, on the occasion of the third joint meeting of the four European early phase clinical drug development associations – namely from Belgium (BAPU), from France (Club Phase 1), Germany (AGAH) and from the United Kingdom (AHPPI). The godparents were Prof Dr Jan de Hoon (BAPU), Dr Y Donazzolo (Club Phase 1), Prof Dr H Sourgens (AGAH) and Dr P Dewland (AHPPI). The aims of the Federation are to promote European Clinical Pharmacology by international meetings, gaining recognition as a European stakeholder in international and national regulatory discussions and to establish an international post-graduate training programme in this area of study.
Over the past four years there have been three scientific meetings involving contributions from all participating associations (Berlin in 2011, Nice in 2013 and Brussels in 2015) with the next being planned for 2017 in the UK. Successful contributions have included revisions to the Clinical Trial Regulation coordinated by the EMA. As a recognised EU stakeholder meetings were attended by EUFEMED committee members.
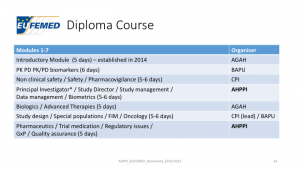
The first modules for EUFEMED’s Diploma in Clinical Pharmacology are scheduled for the fourth quarter of 2016. This is truly international in the content of its syllabus and national delivery by all EUFEMED member associations.
Written by Dr Mike Hammond, Principal of Clinical Quality Management Solutions. Mike graduated in Pharmacology and has a PhD from the University of London. He has held various career positions in drug discovery with US, European and Japanese pharmaceutical companies. He currently works on the Quality and Regulatory aspects of development and commercial exploitation of novel therapies. He is a committee member of the AHPPI Committee.
